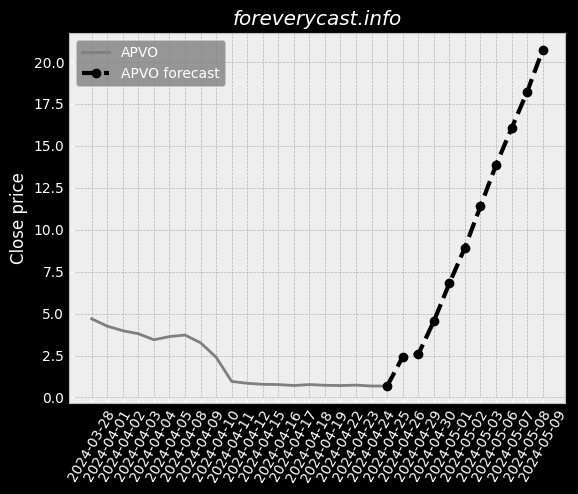
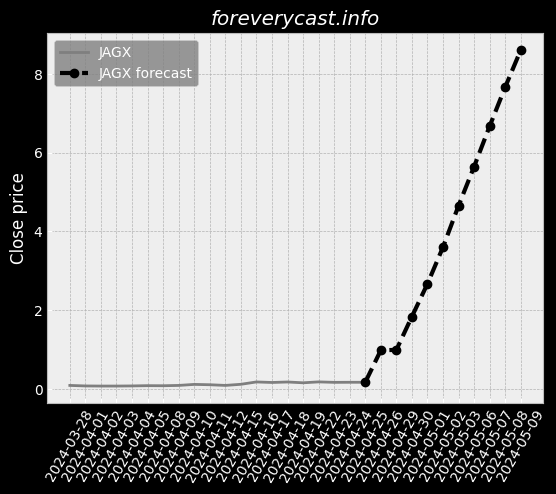
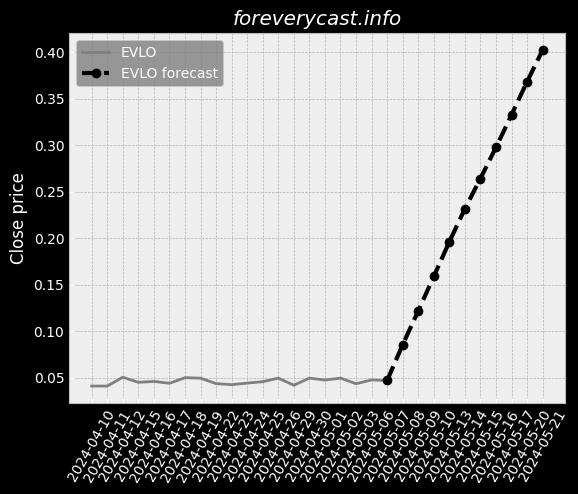
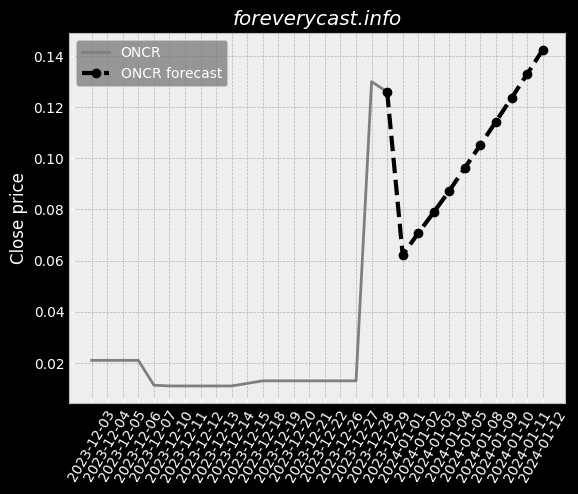
Co-administration of ImmTOR with an auto-antigen, viral vector, or therapeutic enzyme. Approximately 41% of patients in the Phase 2 COMPARE trial had visible tophi at baseline. SEL-212 ComponentsSEL-212 consists of ImmTOR co-administered with pegadricase. All SAEs were successfully treated without further issues. IGAN BiosciencesIn October 2020, we entered into the IGAN Agreement. Under this arrangement, 3SBio has agreed to supply us with pegadricase. The FDA adjusts the PDUFA user fees on an annual basis. A biological product can also obtain pediatric market exclusivity in the United States. As of December 31, 2020, we had an accumulated deficit of $404.6 million. A similar framework is in place in the EU. Preclinical development is costly and inherently uncertain. We may also disclose interim data from our clinical trials. Further, we may be exposed to fluctuations in the value of the local currency in China. We generally contract with third parties for the disposal of these materials and wastes. Termination of a necessary license could have a material adverse impact on our business. We may not have sufficient resources to bring these actions to a successful conclusion. This can be expensive, particularly for a company of our size, and time-consuming. Also, the expected benefit of any acquisition may not materialize.